Fda Booster Shot | L9gc3tbwt3wtcm
A panel of outside experts for the Food and Drug Administration have unanimously recommended an emergency use. The FDA panels vote against authorizing third shots of Pfizers COVID vaccine for healthy Americans aged 16 and over may derail the Biden administrations booster-shot plan.
The goal is for people to start receiving a COVID-19 booster shot beginning in the fall with individuals being eligible starting 8 months after they received their second dose of an mRNA vaccine either Pfizer-BioNTech or Moderna.

Fda booster shot. This is subject to authorization by the US. A Food and Drug Administration advisory panel overwhelmingly voted in favor of Pfizer-BioNTechs Covid-19 booster shots on. Regulators havent verified all the available data.
FDA is conducting an independent evaluation. The FDA staff declined to take a stance on whether to back booster shots of Pfizers Covid-19 vaccine saying US. A Food and Drug Administration advisory panel voted not to green-light COVID-19 booster shots Friday saying more data is needed before it can recommend a third dose to people 16 years old and up.
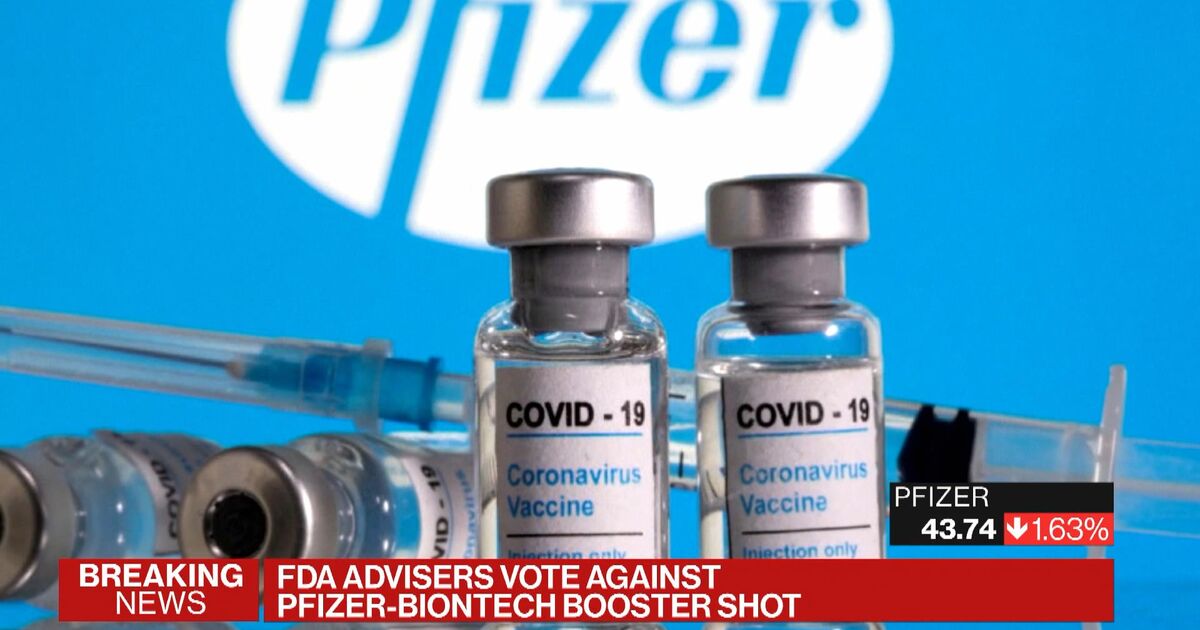
FDA committee votes against Pfizer booster shots for most Americans. Food and Drug Administration and recommendation by CDCs Advisory Committee on Immunization Practices ACIP. The FDA may buck the committees vote and approve the shots regardless within a matter of hours or days and officials with the Centers for Disease Control and Prevention CDC are scheduled to meet next week to discuss distribution plans for the booster doses suggesting that the committees vote was a speed bump on the road to approval rather than a full stop.
A panel of expert outside advisers to the US. FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third booster dose of Comirnaty COVID-19 Vaccine mRNA. Food and Drug Administration voted on Friday to recommend COVID-19 vaccine booster shots for Americans 65 and older and those at high risk of illness.
FDA advisers endorse Pfizer booster shots for elderly and high-risk Americans. Overall data indicate that. Two senior officials have resigned from their positions within the FDA over frustrations with the Biden administrations plans to move forward with recommending COVID-19 booster shots.
Fda Sounds Skeptical Note On Pfizer Booster Shot Ahead Of Key Vote Politico
Fda Releases Pfizer Covid 19 Booster Shot Data Before Panel Review

Fda Panel To Review Pfizer Booster Shots Kron4

Fda Panel Rejects Booster Shots For The General Public Quartz
Israel Said Worried Fda Limit On Booster Shots Will Hamper Its 3rd Shot Campaign The Times Of Israel
U S Fda Vaccine Advisers Vote In Favor Of Covid 19 Booster Shot For Those 65 And Older Reuters
/cdn.vox-cdn.com/uploads/chorus_image/image/69873701/GettyImages_1234851151_copy.0.jpg)